Lead-Acid vs. Lithium-Ion Batteries: Key Differences & Best Uses
June 13, 2025 2025-06-24 14:13Lead-Acid vs. Lithium-Ion Batteries: Key Differences & Best Uses
Lead-Acid vs. Lithium-Ion Batteries: Key Differences & Best Uses
Whether you’re working on an engineering assignment or tackling real-world challenges in the industry, understanding battery technology is crucial. Lead-acid and lithium-ion batteries are two of the most widely used energy storage solutions, each playing a vital role in powering vehicles, industrial systems, and renewable energy applications.
Lead-acid batteries, developed in the mid-19th century, have long been the preferred choice for automobiles, backup power, and industrial machinery due to their reliability and affordability. Their ability to deliver high surge currents makes them ideal for applications requiring a quick and powerful energy supply.
On the other hand, lithium-ion batteries, introduced in the late 20th century, have revolutionized energy storage with their lighter weight, higher efficiency, and faster charging. These features make them the go-to option for electric vehicles, portable electronics, and large-scale renewable energy systems.
But which one is better? The answer depends on factors like energy density, cycle life, charging speed, and overall cost. Exploring these key aspects helps engineers determine the best battery technology for specific applications, shaping the future of energy storage and power systems.
Table of Contents
Lead-Acid vs. Lithium-ion Batteries: Key Differences
Here are the eleven key differences between Lead-Acid and Lithium-Ion batteries:
1. Material Composition and Chemistry
Lead-acid and lithium-ion batteries operate on similar principles, where chemical reactions drive the movement of ions between the anode and cathode, creating an electrical flow. However, the materials used in each type of lead to substantial differences in performance, weight, and durability.
- Lead-Acid Batteries:These batteries rely on lead dioxide as the positive electrode and sponge lead as the negative electrode, with sulfuric acid as the electrolyte. The reaction between the lead plates and sulfuric acid generates electrical energy. This straightforward construction has made lead-acid batteries economical and reliable for many years. However, the use of heavy lead contributes to their significant weight and bulk.

- Lithium-Ion Batteries: Typically, lithium-ion batteries use a lithium oxide compound for the cathode, carbon for the anode, and a lithium salt solution as the electrolyte. Lithium’s light weight and high reactivity allow these batteries to store substantial amounts of energy in a relatively small, light package. The use of lithium also allows for faster ion movement, which contributes to improved charging and discharge rates.

2. Energy Density and Weight
Energy density measures the amount of energy a battery can store in a given weight or volume, which is critical for applications where weight and space are limited.
- Lead-Acid: Lead-acid batteries have an energy density of approximately 30-50 watt-hours per kilogram (Wh/kg). To match the capacity of a lithium-ion battery, lead-acid batteries need to be larger and significantly heavier. This makes them less ideal for mobile applications and limits their use in scenarios where space and weight are constraints, such as in electric vehicles (EVs).
- Lithium-Ion: Lithium-ion batteries can reach energy densities between 100-265 Wh/kg. This means that a lithium-ion battery can store two to five times as much energy per kilogram as a lead-acid battery. This high energy density makes lithium-ion batteries preferable for EVs, consumer electronics, and any application where space and weight are critical considerations.
3. Cycle Life and Durability
Cycle life, or the number of charge and discharge cycles a battery can undergo without significant degradation, is crucial for applications that require frequent battery cycling.
- Lead-Acid: Lead-acid batteries typically support around 300-500 cycles before performance deteriorates. Their lifespan depends heavily on factors such as depth of discharge (DoD) and maintenance practices. If lead-acid batteries are discharged to more than 50% of their capacity frequently, their life span reduces significantly. Proper maintenance, such as avoiding complete discharge and keeping the batteries charged, can extend their life, but only to a limited extent.
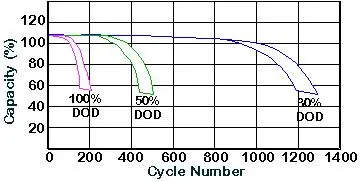
Lithium-Ion: Lithium-ion batteries, on the other hand, can withstand 2,000 to 5,000 cycles or more, depending on their design and application. They’re more resistant to frequent cycling and high depth of discharge, making them ideal for applications like renewable energy storage or EVs, where they are frequently charged and discharged. Their durability results in a longer overall lifespan and reduced need for replacements, which compensates for their higher initial cost.
✅Also read: Series & Parallel Hybrid Vehicles: Are they Outsmarting EVs?
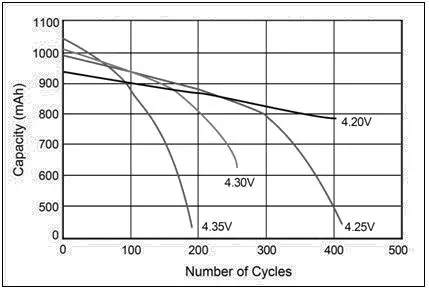
4. Depth of Discharge (DoD) and Usable Capacity
DoD refers to how much of a battery’s capacity can be used without negatively impacting its lifespan. Batteries with higher DoD allow users to make the most of their capacity without needing frequent recharges.
- Lead-Acid: For lead-acid batteries, the recommended DoD is around 50%. Discharging them beyond this point reduces their cycle life, making them less effective in applications requiring frequent deep discharges. This shallow DoD limits the practical usable capacity of lead-acid batteries, making them more suited to backup applications where they stay close to full charge.
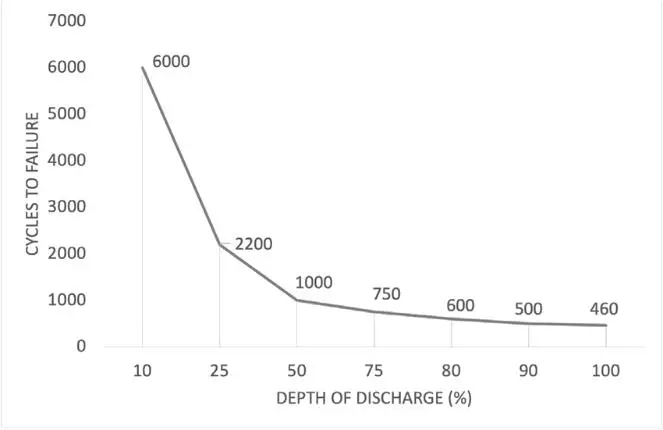
Lithium-Ion: Lithium-ion batteries generally support a much deeper DoD, with many models allowing up to 80-100% discharge. This means that users can utilize more of the battery’s capacity in each cycle without harming its overall lifespan, making it particularly useful for EVs and renewable energy systems that need sustained energy over extended periods.
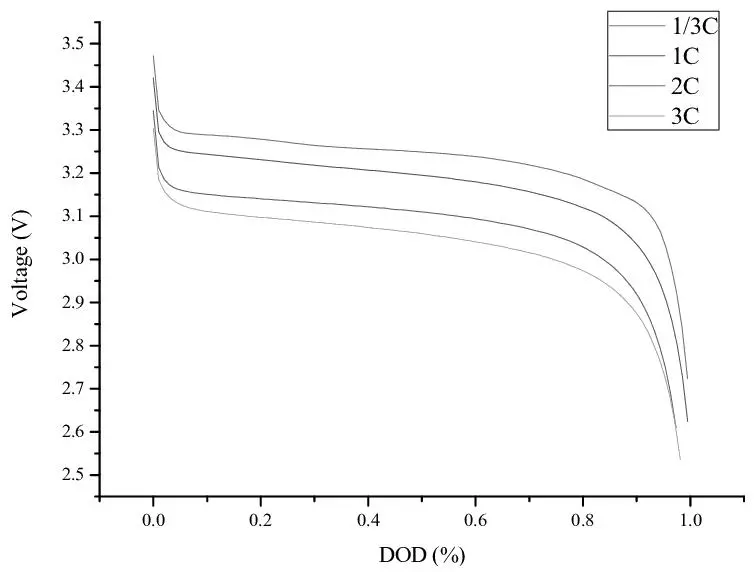
5. Charging Speed and Efficiency
Charging speed is critical in applications where turnaround times are important, such as EVs or devices needing frequent recharges. Efficiency, or the ratio of energy input to usable energy output, also affects overall performance and power consumption.
- Lead-Acid: These batteries take longer to charge, typically requiring 8 hours or more for a full recharge. They are also less efficient, with around 80-85% energy efficiency, meaning some energy is lost as heat during charging and discharging. This slow charging makes lead-acid batteries less practical in scenarios requiring rapid recharge cycles, such as emergency power systems or off-grid solar setups.
- Lithium-Ion: Lithium-ion batteries charge much faster—often within 1-3 hours, depending on their size and charging setup. They’re also more energy-efficient, with efficiencies around 95-98%, resulting in less energy wasted as heat. Their fast-charging capability is essential for EVs and consumer electronics, where minimal downtime is crucial.
6. Maintenance Needs
Maintenance affects both the convenience and the long-term cost of battery ownership.

- Lead-Acid: Lead-acid batteries, especially flooded types, require regular maintenance to ensure optimal performance. This includes adding water to maintain electrolyte levels and keeping the battery charged to prevent sulfation. Without proper maintenance, lead-acid batteries deteriorate faster and may need frequent replacements.
- Lithium-Ion: Lithium-ion batteries are generally maintenance-free. They don’t need water refills or other regular upkeep. Modern lithium-ion systems are equipped with a battery management system (BMS) that regulates charge and discharge levels, ensuring the battery operates safely and efficiently. This low-maintenance nature reduces operational costs, and the time required for upkeep.
7. Temperature Tolerance and Performance
Temperature has a significant impact on battery performance and safety, particularly in extreme environments.

- Lead-Acid: Lead-acid batteries perform better in cold temperatures compared to lithium-ion batteries, although their efficiency still declines as temperatures drop. High temperatures, however, accelerate the degradation of lead-acid batteries, shortening their life.
- Lithium-Ion: Lithium-ion batteries are more sensitive to temperature extremes, especially high temperatures, which can lead to thermal runaway—a self-sustaining heat reaction that can cause the battery to catch fire or explode. They may also lose capacity in extremely low temperatures. To manage these risks, lithium-ion batteries often require thermal management systems, particularly in high-stakes applications like EVs and energy storage.
8. Safety and Risk Management
Safety concerns vary between battery types, particularly in cases of overcharging, physical damage, or extreme temperatures.
- Lead-Acid: These batteries are generally safe but can release hydrogen gas when overcharged, which is flammable and can be hazardous without proper ventilation. Acid leaks, if they occur, are corrosive and require careful handling and clean-up.
- Lithium-Ion: Lithium-ion batteries, while safer in certain respects, pose risks of thermal runaway, which can result in fires if the battery is damaged or mishandled. Advanced BMS technology and safety features like thermal cutoffs and pressure release valves help mitigate these risks, but lithium-ion batteries still require careful usage and adherence to manufacturer guidelines to ensure safe operation.
9. Environmental Impact and Recycling
Recycling practices and environmental impacts play a crucial role in battery technology sustainability, given the materials and processes involved in production and disposal.

10. Applications and Suitability
- Lead-Acid: Lead-acid batteries have an established recycling infrastructure, with over 95% of their components being recyclable as per Canada Renewable Association research. However, lead is toxic, and improper disposal or recycling can lead to environmental contamination. Proper recycling of lead-acid batteries is critical to minimizing their environmental footprint.
- Lithium-Ion: The recycling infrastructure for lithium-ion batteries is still developing, and while they contain fewer toxic metals, the extraction of lithium and other elements poses environmental and ethical challenges. Research is ongoing to improve lithium-ion recycling rates, and as demand grows, more efficient recycling methods are expected to emerge.
➡️Also Read: The Key Electric Vehicle Components – EV Parts & Its Functions
The choice between lead-acid and lithium-ion batteries often comes down to application requirements, cost constraints, and energy needs.

- Lead-Acid: Due to their low cost and robustness, lead-acid batteries are suitable for stationery and backup power applications, such as uninterruptible power supplies (UPS), inverters, and grid energy storage. They’re also widely used in automotive starter batteries, where they can reliably provide the high initial power needed to start an engine.
- Lithium-Ion: With higher energy density, faster charging, and longer cycle life, lithium-ion batteries excel in high-demand applications such as EVs, portable electronics, and renewable energy storage. Their ability to sustain frequent deep discharges makes them ideal for continuous use applications, where consistent, high-quality power is essential.
11. Safety and Risk Management
- Lead-Acid: Lead-acid batteries are relatively stable and safe when used appropriately. However, they can release hydrogen gas during charging, which poses an explosion risk if not properly vented. Mishandling or overcharging can also cause acid leaks, which are corrosive and can damage equipment or pose safety hazards.
- Lithium-Ion: Lithium-ion batteries require a robust battery management system (BMS) to manage risks associated with overcharging, overheating, and short circuits. While generally safe, they can experience thermal runaway if damaged or improperly handled, leading to potential fire hazards. Advances in technology have improved safety, but careful management and adherence to usage guidelines are still critical.
Lead-Acid vs. Lithium-Ion Batteries: 10 Key Differentiating Factors
We have summarized 10 key differentiating factors between Lead-Acid and Lithiom-ion batteries in a table format:
Conclusion
In summary, lead-acid batteries are an economical choice for short-term or low-cycle applications, such as backup power. Lithium-ion batteries, however, are more suitable for high-performance, high-cycle applications like EVs and renewable energy systems. While the upfront cost for lithium-ion is higher, the long-term savings due to durability, efficiency, and minimal maintenance can justify the investment in scenarios demanding frequent power cycling and high efficiency. Each type of battery has carved out a unique place in the energy storage landscape, and advancements in both technologies continue to expand their applicability.
FAQs on Lithium-ion vs Lead-acid Batteries
Prateek Potnis
Related Posts
What is PLM? – Product Lifecycle Management [Detailed Guide]
Top AutoCAD 3D Commands & Shortcuts with Examples
What is Engineering Mechanics? – Introduction 101
How to Create Stitch Weld in Weldments using Autodesk Inventor?
How to Create Blend Curve on Surface using Siemens NX?
Find
Categories
Latest Posts
Popular Tags